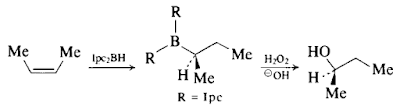
- To a stirred suspension of diisopinocampheylborane (50mmol) (1) in tetrahydrofuran (18 ml) is added 4.5 ml of (Z)-but-2-ene.
- The reaction mixture is stirred at 25 °C for 4.5 hours.
- The solid diisopinocampheylborane disappears and the formation of the trialkylborane is complete.
- The organoborane is treated with 4 ml of methanol, followed by 18.3 ml of 3 m sodium hydroxide and the careful addition of 20 ml of 30 per cent hydrogen peroxide, maintaining the temperature of the reaction below 40 °C.
- The reaction mixture is further stirred at 55 °C for 1 hour, cooled, and extracted with ether (3 x 50 ml).
- The extract is washed successively with water (2 x 25 ml) and brine (3 ml) and dried over magnesium sulphate.
- The organic layer is care-fully fractionated to provide butan-2-ol, b.p. 96-98 °C, 2.9 g (73%), purity > 95 per cent. The last traces of impurities are removed by preparative g.l.c. (2) to yield (R)-butan-2-ol, [a]£ 3 -13.23° (neat), ee 98.1 per cent.
Notes to keep in mind:
1. ( + )-a-plnene [a]£ 3 + 47.1 ° (neat), 92 per cent ee, distilled from a small excess of lithium aluminium hydride and stored under nitrogen, is used for the preparation of Ipc 2 BH.
2. For preparative g.l.c. a 1.8 m x 12.7 mm column packed with 10 per cent Carbowax 20m on Chromosorb W is used.
No comments:
Post a Comment
We specialize in producing high value chemicals. Besides our regular products, we strive to develop new products based on customer’s requirements. Our R&D center plays crucial role in handling complex chemistries and developing newer technologies. We respect intellectual property rights and have confidentiality agreement with various multi national companies. We undertake contract manufacturing of fine chemicals and advance intermediates of API’s.