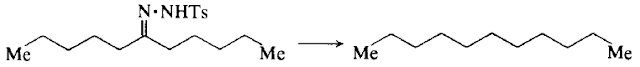
STEP 1: To a slurry of undecan-6-one toluene-p-sulphonylhydrazone (5.08 g, 15 mmol) (1) in 50m1 of glacial acetic acid is added sodium borohydride pellets (c. 5.67 g, 150 mmol, 24 pellets) (2) at such a rate that foaming is not a problem (c. 1 hour).
STEP 2: The solution is stirred at room temperature for 1 hour and then at 70 °C for 1.5 hours.
STEP 3: The solution is then poured into crushed ice, made basic with aqueous sodium hydroxide and extracted with three portions of pentane.
STEP 4: The pentane solution is dried and concentrated in a rotary evaporator, and the residue distilled at reduced pressure (Kugelrohr apparatus) to obtain 1.96 g (84%) of undecane.
STEP 5: Undecane has b.p. 87 °C/20 mmHg.
Notes to keep in mind:
1. The hydrazones are prepared by the following general procedure.' The carbonyl compound and a 10 per cent molar excess of toluene-p-sulphonylhydrazine in absolute ethanol (c. 2 ml per gram of carbonyl compound) are heated on a steam bath until a clear solution results (15 minutes). Cooling affords crystalline products in good to excellent yields. Recrystallisation is accomplished from ethanol or aqueous acetone. For hindered ketones periods of up to 14 hours of reflux are suggested.
2. Obtainable from Alfa Inorganics.
No comments:
Post a Comment
We specialize in producing high value chemicals. Besides our regular products, we strive to develop new products based on customer’s requirements. Our R&D center plays crucial role in handling complex chemistries and developing newer technologies. We respect intellectual property rights and have confidentiality agreement with various multi national companies. We undertake contract manufacturing of fine chemicals and advance intermediates of API’s.